
Written By: Max Perelman
As CEO, I am extremely proud to be marking Biomeme’s 10th anniversary as a company — a significant milestone.
In 2012, Marc DeJohn, Jesse vanWestrienen and I started Biomeme. In a decade, we went from our first prototype PCR thermocycler using a travel hair dryer and an iPhone 4 to being one of the nation’s leading biotechnology companies offering streamlined solutions for molecular* detection. Our focus was simple: to empower anyone, anywhere with the tools of a molecular lab in the palm of their hand.
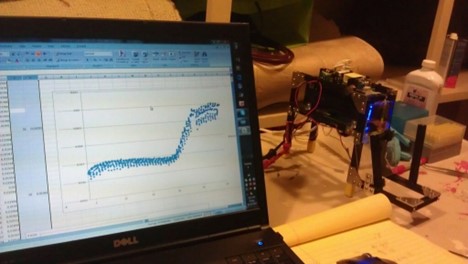
Our first successful PCR amplification plot is shown on a laptop, with the first Biomeme prototype Marc assembled from a travel hair dryer and iPhone 4.
Rewind back to 2012…Dr. Eric Topol had just published The Creative Destruction of Medicine: How the Digital Revolution Will Create Better Health Care. Innovators, such as Alivecor, Cellscope, and MobileODT had just started to test the boundaries of what a smartphone could do from a scientific/diagnostic perspective, but no one was maximizing the use of the iPhone sensors & peripherals** to do real-time DNA amplification, detection, and quantification. We started with the instrument challenge first – could we replicate the basic capabilities of a real-time PCR thermocycler that one might find in an advanced molecular biology lab, by only using a simple add-on to a smartphone? But wait, why did we even want to use a smartphone?
At the time, the smartphone was fast becoming the center of many of our most complex social and technical interactions. Everyone had one (or would soon have one) and they just worked. There were no complex instruction manuals or specialized trainings – even my parents could buy an iPhone and quickly start using the new gadget.
So, why couldn’t the smartphone be the primary user interface to mediate arguably one of the most complex laboratory techniques?
Marc and Jesse were the technical experts, but in my mind, I was the target user because if I could do it then anyone could. We iteratively improved the commercial instrument – the first system could detect one target in each of its three reaction wells, so we named it the one3. Why three wells, you ask? We went back and forth on this for the MVP (minimum viable product) and decided the fewest reactions a scientist would need would be three because they would need to test the sample, as well as run a positive control and a negative control. The next system could detect two targets per well, so we called it the two3. Prototyping within the mobile iOS environment was fast and we’d essentially outsourced the majority of our sensors to Apple, but our dependence on the specific iPhone form factor and the black box camera sensor made long-term instrument development impossible. Therefore, our future instrument iterations have decoupled from the smartphone, and we can now control the systems via Android/iOS smartphone, tablet and/or PC.
We’d solved the problem of the thermocycler, laptop controller, gel electrophoresis setup***, and electricity requirement… simplifying everything and miniaturizing it into a unit the size & weight of a soda can.
After developing the mobile thermocycler, we quickly realized the instrument was meaningless if you didn’t have a simple, easy method to prep your samples.
There were no good options for simple, fast, and effective nucleic acid extraction & purification (i.e., sample prep). Unless you could get at the DNA/RNA in your sample and remove the extraneous junk, users were relegated to transporting their samples back to a central lab for extraction. Back at the lab, a trained molecular biologist followed a complex, multi-hour process typically involving the mixing and transferring of various chemicals with lab pipettes and using a series of benchtop equipment including a vortexer, heat block, and centrifuge.
Therefore, we made solving the sample prep challenge a key priority for the team. Marc and Jesse came up with an elegant solution centering around an adapter for a standard 1mL syringe that could be paired with a series of buffers – originally in color-coded tubes (demonstrated by my daughter 7 here), and now consolidated into a single cartridge, as shown here. Importantly, as part of their R&D, our team of biochemists have also spun out collection & storage solutions to inactivate pathogens and stabilize the DNA/RNA in a range of sample types.
Our next challenge became apparent when one of our collaborators, running our Biomeme equipment high up in the Andes, had issues with her PCR test chemistries which must maintain an unbroken cold chain.
She’d meticulously packed her PCR test reagents in coolers on donkeys, but somehow, they’d gone bad, ruining her field research. PCR Lyophilization (i.e., freeze-drying) was the 3rd piece of the puzzle. Stored as separated components in lab freezers and shipped cold (often on dry ice), the multiple reagents that compose a PCR assay test can be made shelf-stable if you lyophilize them. But this is not easy, and few have developed formulations or processes that yield a dried product with the same performance as it’s wet alternative. After years of iteration, we’ve perfected solutions that last up to 3 years at 30oC. And we can dry the pre-mixed assays in a range of form factors.
This may seem trivial, but when you’ve trekked to a remote mountain top, are ankle deep in bat guano in the Congo, or are running the next batch of COVID tests for the cast of Stranger Things… this all just must work.
And after it just works, that test data needs to be backed up and accessible in the cloud from anywhere. After the mobile app, our software engineers expanded to develop a cloud portal and web API with the vision of receiving data from thousands of distributed mobile PCR systems around the world. During the COVID pandemic, these capabilities were critical to enable diagnostic lab directors, technical supervisors, and quality personnel to remotely monitor in real-time the data coming in from hundreds of test sites around the country. And then integrate that data seamlessly into multiple lab information and epidemiological reporting systems.
2020 turned the world upside down and marked a period of rapid, yet tortuous, growth for Biomeme. The 8 years prior had laid the infrastructure and foundation to meet the unprecedented needs of the global pandemic, but it has not been easy.
For over two years, we’ve experienced (and continue to experience) a seemingly endless string of challenges. And the team has made so many sacrifices.
As a physical manufacturer of diagnostic products, we didn’t have the luxury of moving everyone remote during the pandemic. This created an enormous amount of stress on the team, especially in the early days when there were no COVID vaccines or treatments. I remember making my first call to notify one of our staff that she’d been exposed to a colleague who had tested positive. She started crying and asking if her children would be okay. Our team braved the dangers of a novel virus with unknown health effects and no vaccine in sight, to come into the office, manufacture, and ship items around the world to the frontline testing staff fighting the pandemic.
I remember a single week in 2020 when we faced massive shortages of every raw material, protests outside our facility, an outbreak of COVID within one of our manufacturing shifts, and massive storms including a tornado. The team worked through ALL of this.
What keeps me going are the calls I get from groups telling me about the lives we have saved, the outbreaks we have helped to end or prevent, the businesses we have enabled to reopen.
I would like to say thank you to everyone within the Biomeme family who have made it possible for us to have achieved so much these past 10 years. And I look forward to working with all of you on this next leg of our adventure.
Cheers to 10 years!

Max Perelman
Biomeme Co-Founder, President and CEO
*The industry term “molecular” denotes DNA/RNA and usually refers to Real-Time PCR, as differentiated from other technologies that detect cultures, antigens, antibodies, toxins, heavy metals, etc.
**We were using the touchscreen as the primary user interface, lightning connector to communicate with the thermocycler dock, front facing camera as a QR code reader for tests, back camera as a fluorimeter to detect the polymerase chain reaction (PCR), internal processors to analyze data and generate test results, GPS to tag location data to test results, WiFi radio to transmit data off the system, and more.
***While some companies, such as OpenPCR and MiniPCR, have focused on standard PCR which only amplifies DNA/RNA and therefore requires additional equipment to visualize the results, Biomeme’s first instrument ran real-time PCR which combines both amplification and detection in one.
Get the latest tips from Biomeme shipped right to your inbox

As CEO, I am extremely proud to be marking Biomeme’s 10th anniversary as a company — a significant milestone. In 2012, Marc DeJohn, Jesse vanWestrienen and I started Biomeme. In a decade, we went...

We’ve talked a lot about the immense growth has seen in recent years. Throughout our growth we’ve had people from all walks of life, all over the world, and of all kinds of perspectives work...

Biomeme’s move into its new corporate headquarters at Netrality's 401 North Broad Street, will begin an exciting new chapter for our company. Our new headquarters were announced earlier this year and...
401 North Broad St Suite 222 Philadelphia, PA 19108