 Our Approach
Our Approach
From the battlefield to the kitchen table and everywhere in between, antibiotic resistance is cropping up in—and being battled—in new and evolving ways. Antibiotic resistance (ABR) fits under the...
Get the latest tips from Biomeme shipped right to your inbox

It might not seem like warming global temperatures and drug-resistant bacteria have any connection. But, in fact, they do. Antimicrobial resistance (AMR) is one of the world’s wicked problems because...

Overuse of antibiotics has led to a losing battle against mutating, drug-resistant bacteria. A restrained approach to antibiotic prescription will be key to addressing this massive global health...
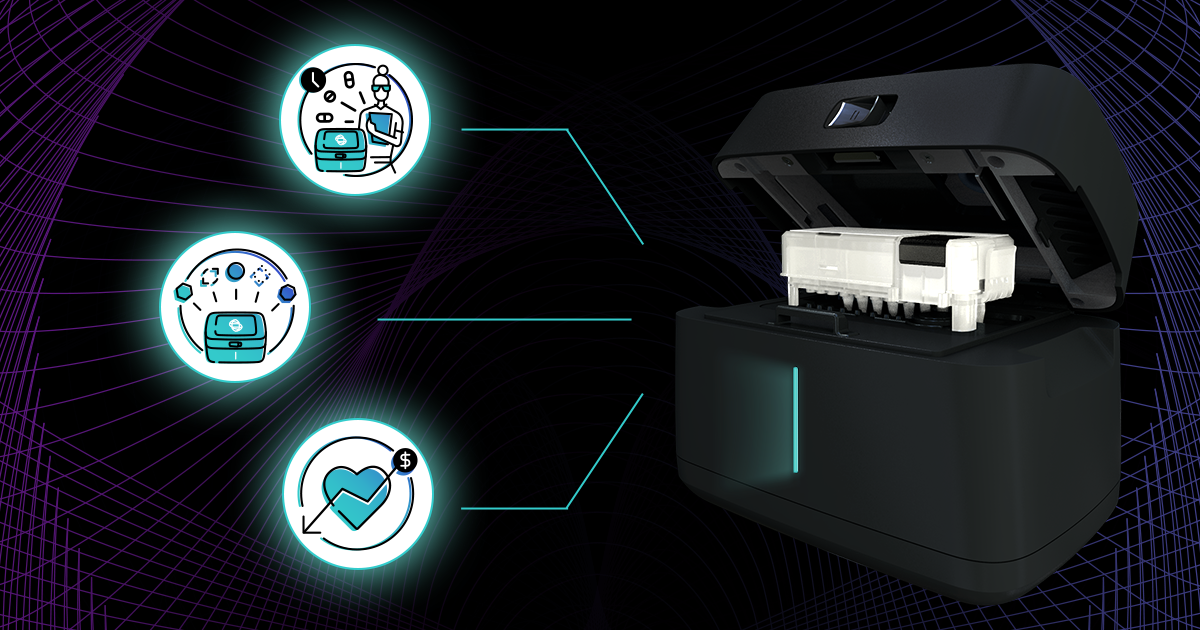
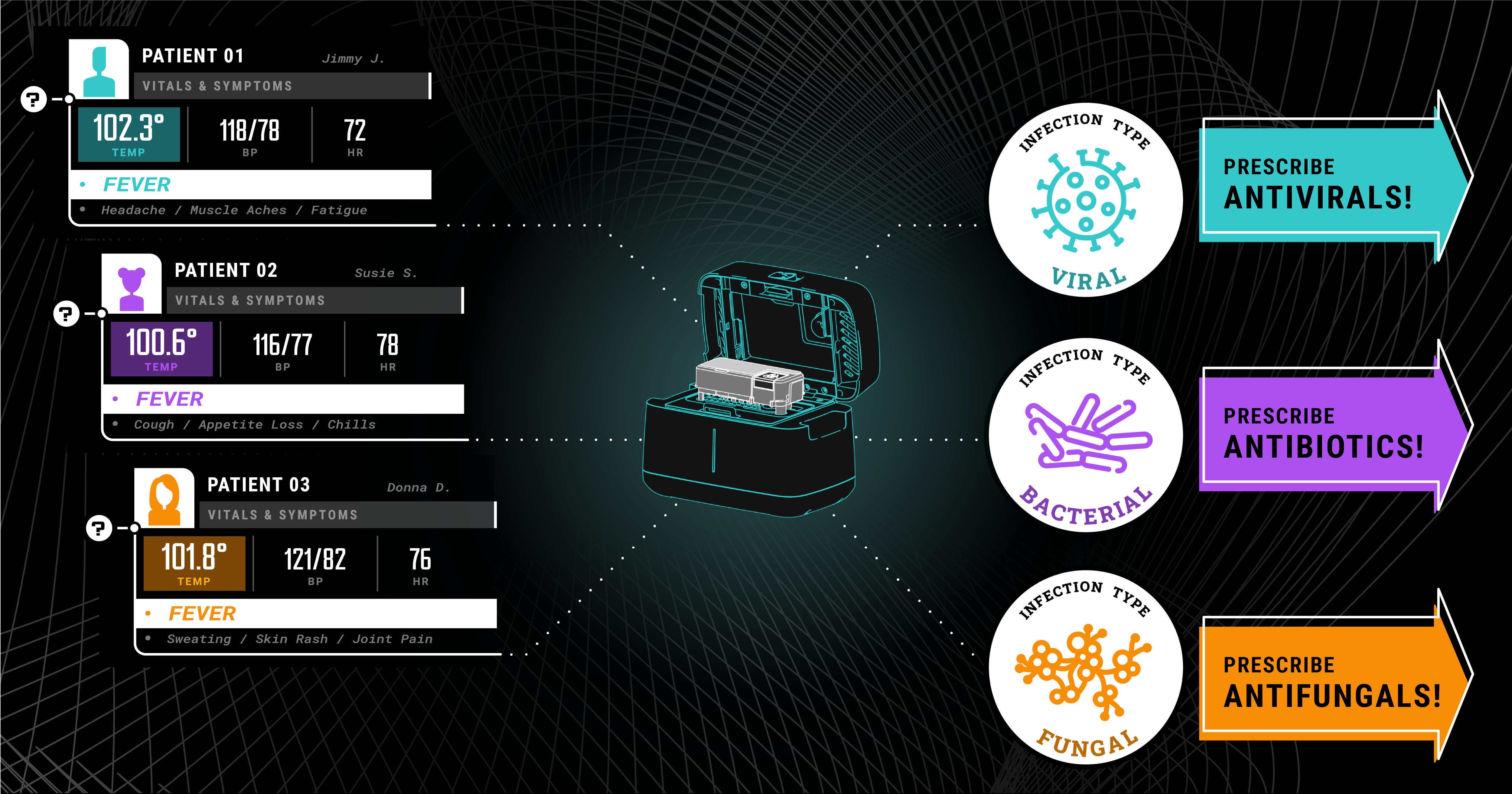
Host response testing allows physicians to quickly rule out entire categories of potential infection to narrow their focus to the likely culprits for an illness. This can enable physicians to make...

Biomeme pioneers a new era in healthcare with the HR-B/V platform. Our host response molecular testing, based on decades of research and collaboration with leading institutions, empowers healthcare...
It’s no secret that there are major issues in the healthcare industry, including breakdowns in doctor-patient communication, delayed test results, and information gaps. Even after consulting with...
.jpeg)
You’re sick. You’ve been sick for a couple of days. You feel awful. You feel so awful that you go see your doctor. They can’t quite figure out what’s the matter, but they prescribe you a general...

After 15 years working in molecular diagnostics, I know how far the science has come and who is doing what in this industry. And right now is an incredible time to be working in the IVD space because...

With the rapid advancements in bioterrorism, the landscape of potential threats faced around the world is evolving. Among the many establishments navigating these challenges, the U.S. State...

Since 2012, Biomeme has been dedicated to addressing global health challenges by developing state-of-the-art platforms and decentralized testing solutions that can detect and identify infectious...