
Written By: Biomeme Staff
At Biomeme, we are passionate about democratizing science and that means occasionally synthesizing the latest academic research into relatively shorter briefs. The question of which type and size of water filter to use is rather more complex than it may appear at first glance -- but super important to achieving desired eDNA yield! Below you’ll find a brief overview of general guidelines when collecting aquatic eDNA, a small summary of where the literature stands on both filter pore size and material, and how Biomeme’s on site sample prep and qPCR platform can help optimize your aquatic ecology workflow.
The capture of eDNA for subsequent qPCR testing has become the gold-standard for aquatic ecologists and wildlife management professionals. Even with the emerging prevalence of eDNA based methods, however, there remains no standardized protocol for the capture and extraction of aquatic eDNA. Indeed, the optimal filter and extraction combination for any particular case depends heavily on source water characteristics, expected present biomass, target, and multiple other factors.
In the last five years there have been exciting studies published and new filter products brought to market. In this blog post you’ll find a summary of the agreed upon guidelines that have emerged from the literature, some things to consider when choosing your filter, and the recommended protocol for using water filters in the larger Biomeme Sample Prep and mobile qPCR thermocycling platform.
- Avoiding contamination is crucial!
- On site filtration is better! Always try to filter water samples within 24 hours.
- Generally, the greater the volume of processed water, the higher the expected eDNA yield!
- Filtration is preferable to precipitation!
There is a general consensus on the above guidelines -- but many plausible options remain regarding actual filter selection. While it is almost always the case that “methods that maximize eDNA recovery in a cost-efficient manner are ideal” (Hinlo et al 2017), maximal eDNA recovery is likely to depend on the unique characteristics of specific experimental design. Besides cost, the eternal trade-off between smaller pore size and increased clogging remains an important consideration.
First, though, it’s important to note from the outset that nominally equivalent pore sizes may not be effectively equivalent across various materials. Hunter et al (2014) report “filters of different materials with identical pore sizes actually have significantly different effective pore sizes” and so “should not be compared as if their particle retention is equivalent”. This difference is under discussed in the academic literature but suggests that a reasonable margin of error should be assumed in all discussions of pore size and material.
Recommended pore sizes in the literature range from 0.2 µm to 1.2 µm. Let’s briefly cover the smaller, larger, and middle points of the spectrum.
Considering 0.2 µm to be “optimal” for eDNA capture yet recognizing it is likely to suffer from considerable clogging in most eDNA use-cases, Turner et al (2014) provide the following formula for isocline calculations for combinations of larger pore size and greater water volume:
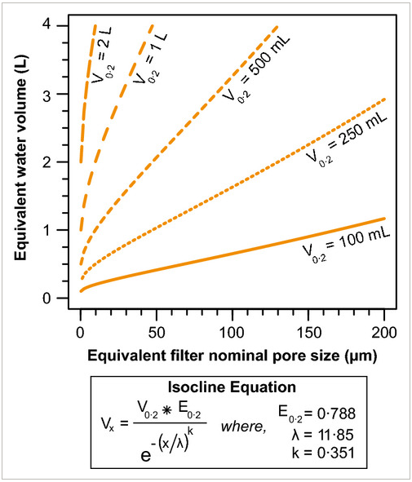
(Turner et al 2014)
At the larger end of the spectrum, Hunter et al (2019) note that because “most eDNA is cell-bound” the larger 1.2 µm filters are “sufficient to capture the cells while simultaneously allowing particulates in the water to pass through”. If targeting macro-fauna in water with higher turbidity, it will likely make more sense to begin with a larger filter.
In classic Aristotelian fashion, multiple studies suggest filters with pore sizes in the middle 0.6 to 0.7 µm range. Eichmiller et al (2015) call 0.6 µm “an optimal balance” and Minamoto (2016) reminds us that because “most eDNA from macro-organisms … measure between 1 and 10 µm in field samples … the use of [a] 0.7 µm pore-size filter is reasonable for collection of eDNA from field samples”.
Whether 0.2, 0.7, or 1.2 µm., though, pore-size is only half the question. Filter material must also be considered. Again there is some disagreement in the literature about which filter material type produces the greatest eDNA yield. The effect of filter material on yield is almost certainly interactive with extraction and preservation methods and dependent on water and sample characteristics. Nonetheless, there is support reported for both glass fiber and cellulose nitrate filters:
- Glass Fiber (note, we do not recommend glass filters for our system)
- Generally cheaper than polycarbonate filters
- ‘Depth filter’ which retains particles within the filter matrix as opposed to polycarbonate membrane filters, which retain all particles larger than the pore size on the filter’s surface (Eichmiller 2015)
- Cellulose Nitrate
- Filtration using cellulose nitrate filter paper outperformed other combinations in terms of cost and efficiency of DNA recovery. (Hinlo 2017)
- Cellulose nitrate (CN) reported high eDNA yield when compared with polyethene sulfone (PES), polyvinylidene fluoride (PVDF) and polycarbonate (PC) filters. (Majaneva 2018)
Biomeme’s end-to-end platform is optimized for mobile, on-site sample prep and qPCR. Given the overwhelming importance of minimizing contamination and the clear advantage of filtering samples within 24 hours, our technology is particularly advantageous for aquatic ecologists (and other scientists using eDNA). For use with our system, we recommend the following:
- Sterlitch MCE Membrane Filters
- 1.2 µm pore size
To give you a better idea of how water filters fit in our workflow, here is our recommended protocol for implementing water filters in our system (complete with How-To video).
- Be sure to use with the Homogenization Kit
- Unscrew the 5mL blue capped tube and set the ball bearing in the inverted cap of the tube
- Remove your filter from the filter housing and roll so that the surface containing the majority of the filtered material is facing inwards, then place into the blue-capped tube
- Using a 1 mL transfer pipette, add 2mL of the homogenization buffer (BLB) to the blue-capped tube
- Be sure to spread and flatten your filter against the inside of the the blue-capped tube before placing the ball bearing inside
- Carefully place the ball bearing inside, close the cap and vigorously shake the tube for no less than 1 minute to mechanically disrupt the sample
- Using an additional 1mL syringe, draw up to 1mL of the homogenized solution containing your sample
- Firmly seat the syringe filter onto the syringe tip’s luer lock and then slowly push down on the syringe plunger to filter your sample into the sample prep cartridge
- Dispose of syringe accordingly
- Proceed with sample prep
- Watch the video on M1 Sample Prep here.
Check back soon as we continue our blog series on sample types with whole blood! Better yet, subscribe to get notified when we publish our next blog post...
Carim, Kellie J et al. 2016. “A Protocol for Collecting Environmental DNA Samples from Streams”
Hinlo et al. 2017. “Methods to maximise recovery of environmental DNA from water samples”
Majaneva et al. 2018. “Environmental DNA filtration techniques affect recovered biodiversity”
Thomas, Austen et al. 2019. “A self-preserving, partially biodegradable eDNA filter”
Turner et al. 2014. “Particle size distribution and optimal capture of aqueous macrobial eDNA”
Get the latest tips from Biomeme shipped right to your inbox
Host response testing allows physicians to quickly rule out entire categories of potential infection to narrow their focus to the likely culprits for an illness. This can enable physicians to make...
401 North Broad St Suite 222 Philadelphia, PA 19108