Written By: Biomeme Staff
We are pleased to announce that the U.S. Food and Drug Administration (FDA) amended Biomeme’s SARS-CoV-2 Real-Time RT-PCR Test Emergency Use Authorization (EUA) for use with pooled samples in laboratories that meet requirements to perform high complexity tests for the duration of the declaration. It is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures.
Pooled testing is ideal for testing many samples at once to quickly determine if SARS-CoV-2 viral RNA is detected in a group of people. This workflow is most effective for schools, workplaces, and live events where large groups of people must be screened quickly to minimize the spread of COVID-19 because it:
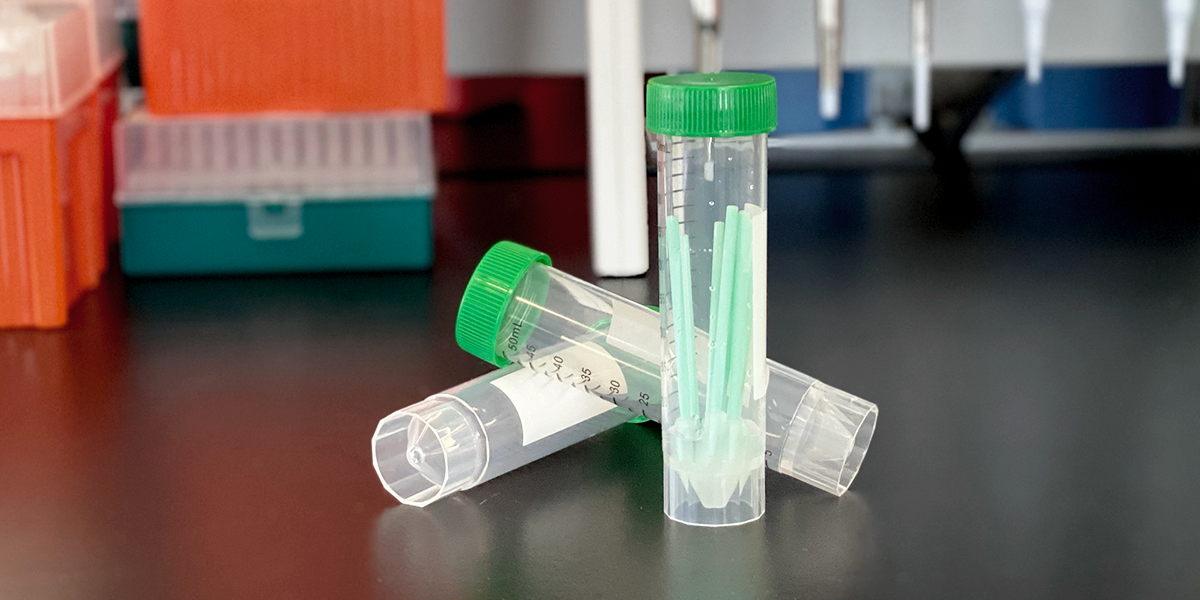
The test is approved for two pooling options: 5X Wet Media pooling and 10X Dry Swab pooling. To accommodate our power users, Biomeme developed our SARS-CoV-2 Pooled Sample Collection Kit for 10X Dry Swab pools allowing users to maximize throughput without sacrificing performance.
Biomeme’s SARS-CoV-2 Pooled Sample Collection Kit includes:
The kit is fully compatible with Biomeme’s SARS-CoV-2 Real-Time RT-PCR Test and our portable Franklin™ thermocycler.
Pooled sample collection increases the number of samples tested per thermocycler per hour from 9 samples to 90 samples.
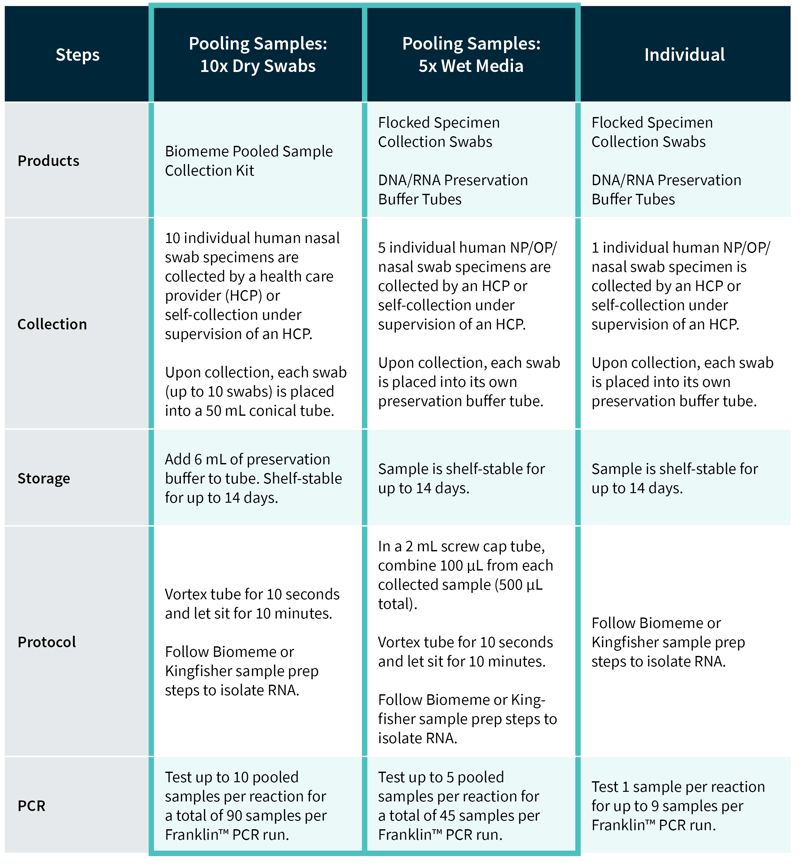
Biomeme’s Pooled Sample Collection Kit improves upon the overall SARS-CoV-2 test workflow. Here’s a comparison table highlighting the differences between Biomeme’s pooled and individual sample collection:
Pro Tip:
If you need the added throughput and cost savings from pooling up to 10 dry swab samples, but you're concerned about having to go back and recollect samples from 10 patients within a Dry Swab Pool that tests positive, then consider collecting 2 samples initially (1 to be pooled and 1 individual to be tested if the pool is positive).
- Go-Strips: Each order consists of a single pouch containing a pre-cut and pre-aliquoted tray of (32) 3-well Go-Strips with void filling caps. Each well contains a 20 µL lyophilized triplex reaction.
- Go-Plates: Each order consists of a single pouch containing a pre-aliquoted 96-well Go-Plate. Each well contains a 20 µL lyophilized triplex reaction.
Each shelf-stable test is a ready-to-use formulation that largely simplifies assay setup because each reaction well already contains the necessary master mix, enzymes, and multiplexed primers and probes. Our test also includes Biomeme’s RNA Process Control (RPC) for RNA extraction and RT-PCR to identify potential inhibition in RNA samples, differences in sample handling, or an issue with the reagents.
Two methods were used to validate the Pooled Sample Collection Kit to prove that the accuracy and sensitivity of Biomeme’s SARS-CoV-2 Real-Time RT-PCR test is not negatively impacted by pooled sample collection.
Validation needed to demonstrate the following:
The validation must demonstrate:
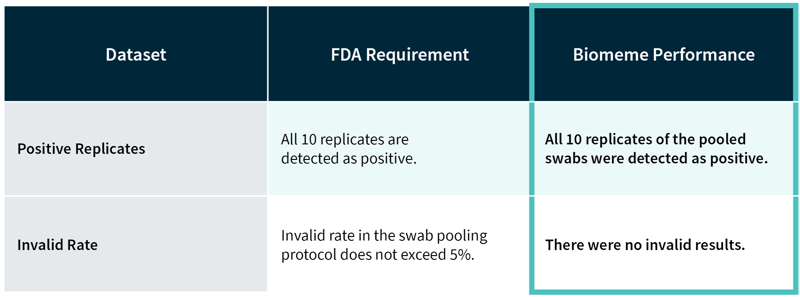
These studies show that the accuracy and sensitivity of Biomeme’s SARS-CoV-2 Real-Time RT-PCR test is not impacted significantly by pooled sample collection.
If you are interested in SARS-CoV-2 Pooled Testing to lower costs and increase throughput without sacrificing accuracy, we recommend contacting our Sales Team.
None of the COVID-19 vaccines on the market protect you 100% against the SARS-CoV-2 virus that causes COVID-19. Although the vaccines decrease the possibility of viral transmission and symptomatic illness, vaccinated individuals can still test positive for the presence of SARS-CoV-2. And it is still important to get tested if you experience COVID-19 symptoms to limit the transmission of the disease.
The CDC recommends you know the following about COVID-19 Vaccines:
- “We are still learning how well vaccines prevent you from spreading the virus that causes COVID-19 to others, even if you do not have symptoms. Early data show that vaccines help keep people with no symptoms from spreading COVID-19.
- We are also still learning how long COVID-19 vaccines protect people.
- We are still learning how many people have to be vaccinated against COVID-19 before the population can be considered protected (population immunity).
- We are still learning how effective the vaccines are against new variants of the virus that causes COVID-19.”
If you have been exposed to someone who has tested positive for COVID-19, follow the CDC (Centers for Disease Control) recommendations to prevent transmission for fully vaccinated or unvaccinated individuals.
Get the latest tips from Biomeme shipped right to your inbox

Biomeme pioneers a new era in healthcare with the HR-B/V platform. Our host response molecular testing, based on decades of research and collaboration with leading institutions, empowers healthcare...
It’s no secret that there are major issues in the healthcare industry, including breakdowns in doctor-patient communication, delayed test results, and information gaps. Even after consulting with...

Biomeme has announced its participation in a collaborative fight against antimicrobial resistance and antibiotic misuse, focusing on better diagnostic testing. With the Antibacterial Resistance...
401 North Broad St Suite 222 Philadelphia, PA 19108