
Written By: Biomeme Staff
Biomeme recently announced the completed acquisition of Predigen. The announcement brings together Predigen's combined decades of host response research with Biomeme's portable PCR test solutions to create a revolutionary diagnostic platform.
Founded in 2016 by internationally recognized Duke Clinicians and scientists, Predigen is a precision medicine company. They are a global leader in the development of host gene expression signatures for use as prognostic, diagnostic, and therapeutic monitoring tools.
Predigen is devoted to advancing the diagnostics field in areas of high unmet need, including, but not limited to, infectious disease, cardiovascular disease, autoimmune and inflammatory disease, and drug response. They have 20 years of research and development at Duke initiated by Defense Advanced Research Projects Agency (DARPA), over $50 million in funding from Department of Defense (DoD), National Institutes of Health (NIH), and Department of Veterans Affairs (VA), and 9 patents at various stages of prosecution.
Predigen’s expertise was in identifying biomarkers that can be used to improve how we diagnose and manage health and disease. However, Predigen lacked the technical solution to measure those biomarkers at the point-of-need.
Biomeme offers a full suite of end-to-end mobile molecular detection solutions that perform to the gold standard used by the world’s most advanced central labs yet require no lab equipment or special experience to use.
Together, we offer an end-to-end solution that measures novel and innovative biomarkers using cutting-edge technology that address significant unmet needs in medicine. After working together for three years, we came to appreciate how closely aligned our mission and values are. Each company complements the other. A merger was the obvious next step.
Many of Predigen’s team members have joined the Biomeme family:
Other Predigen consultants will continue to lend their expertise to Biomeme including Dr. Thomas Burke, Christina Segal, and Grendel Burrell.
Biomeme and Predigen, along with their HR-B/V testing platform and continued host response efforts, were named finalists of The NIH’s Antimicrobial Resistance Diagnostic Challenge (AMR), a $20 million federal prize competition seeking innovative, rapid point-of-care laboratory diagnostic tests to combat the development and spread of drug resistant bacteria.
The partnership and innovative Host Response platform have received continued support over the years from:
We (hosts) exist in a state of balance and homeostasis with our environment. That balance can be disrupted by internal or external stress such as poor nutrition, smoking trauma, or infections. We have also evolved highly sophisticated responses to counteract the stress, restore balance, and maintain health. These responses occur at a molecular level through change in gene expression: genes are turned on or off in a specific manner, serving as a signature for the underlying stress. These signatures are amongst the earliest changes occurring in response to stress and can even be identified before symptoms have begun.
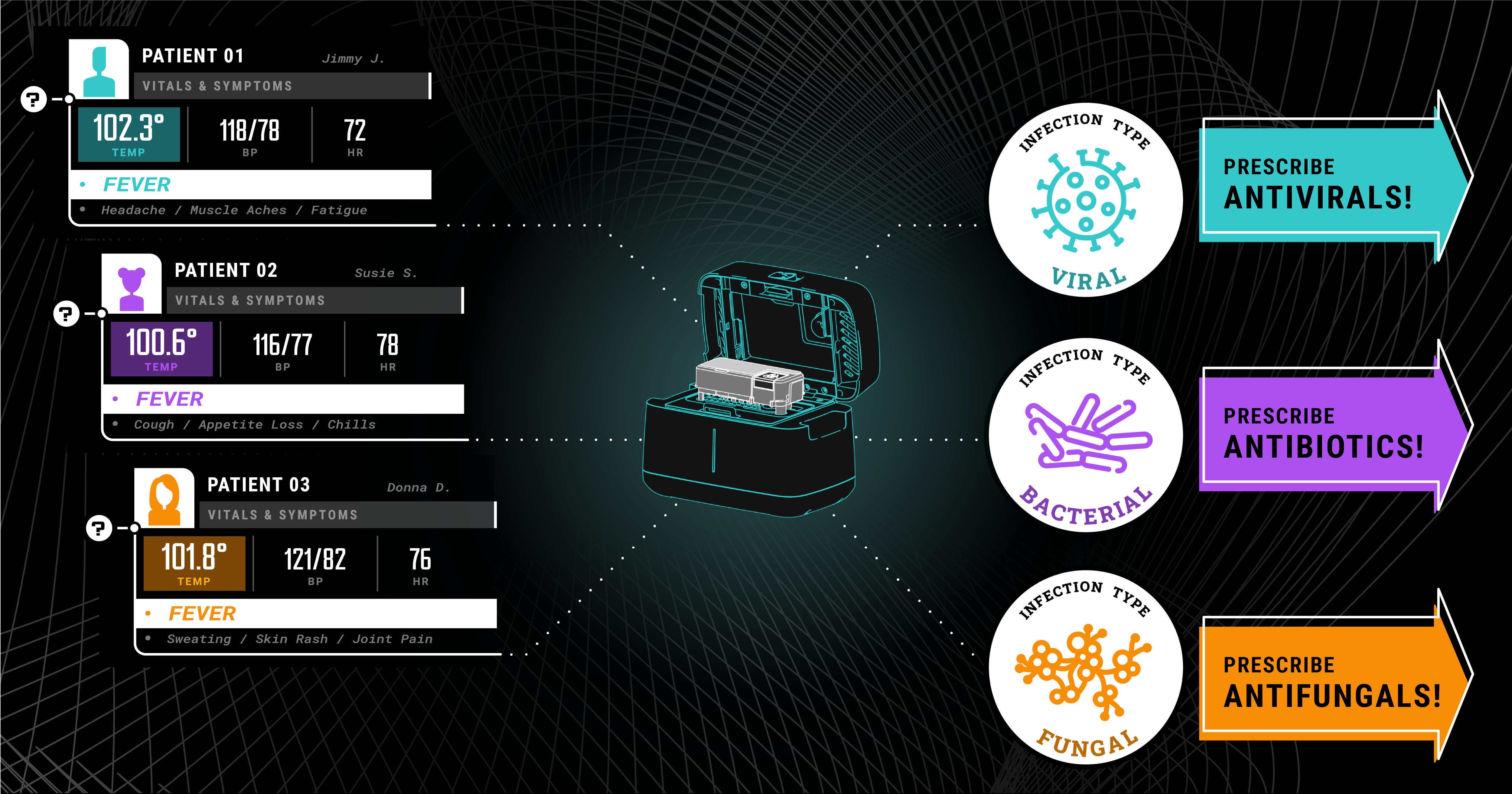
By looking at the expressions of thousands of genes and using advanced machine learning to identify patterns, Predigen has discovered signatures for diseases that are very difficult to diagnose any other way. This includes a gene expression signature indicating the presence of a bacterial infection and another signature for viral infection. These signatures then get translated into diagnostic assays and measured using the Biomeme Platform (e.g., Biomeme’s Host Response Bacterial/Viral (HR-B/V) Test).
Using as little as a drop of blood from a fingerstick, the HR-B/V test can tell your healthcare provider whether your illness is due to a bacterial or viral infection. Armed with that knowledge, they can make more appropriate decisions about prescribing antibiotics. This is one of many examples where we’re not detecting the specific pathogen, rather, we read the telltale signs created by your immune system as it responds to infection.
You can learn more about the new additions to the Biomeme team and their previous publications on Host Response by clicking here.
With Predigen’s deep host response knowledge and Biomeme’s expertise in qPCR consumables, hardware, and software, the new and improved team is equipped to launch their first host response diagnostic platform as early as 2023 in hospitals, ERs and nursing homes. Together, we will chart a path that delivers the next generation in host gene expression-based tests. The two areas of initial focus are the discrimination of bacterial from viral infection (HR-B/V) and pre-symptomatic viral infection (HR-PreV).
Acute Respiratory Infections (ARIs) caused by bacterial or viral pathogens are among the most common reasons for seeking medical care. ARIs are the most common cause of ambulatory visits in US, accounting for 120M visits per year: 72% of visits are unnecessary, and antibiotics are inappropriately prescribed in 50% of cases. Antibiotics do not treat viruses; overuse of antibiotics causes the largest number of drug-related adverse events and drives antimicrobial resistance, which increases costs by $30BB annually.
A diagnostic test, such as Biomeme and Predigen’s Host Response Bacterial/Viral (HR-B/V), can help put an end to this. If you have an ARI, your provider could test a sample of blood and quickly tell whether it is bacterial or viral based on your immune system’s response, before prescribing antibiotics. This ability to determine the precise etiology of respiratory illness will drastically improve personalized healthcare and greatly mitigate antibiotic overuse for millions of people.
Moreover, the ability to distinguish symptomatic from asymptomatic viral infection has immense potential for slowing, treating, and managing infectious disease outbreaks and pandemics. Currently, there is no reliable way to identify pre-symptomatic patients, yet early diagnosis can lead to earlier, more effective therapy as well as quarantine before someone can spread the infection to others. Research to date has shown that the HR-PreV signature detects viral infection up to three days before peak symptoms and before viral shedding is detected. These host gene response signatures can form the basis of novel approaches to early identification and management of emerging viral outbreaks and pandemics for millions of people.
The field of Host Response studies how the body responds to health and disease at the molecular level. Therefore, any condition that triggers a biological response in the form of gene expression is a potential application. The two areas offering the greatest opportunities are infectious and inflammatory diseases. Beyond bacterial and viral infections, there are also opportunities to develop host response tests for malaria, tuberculosis, and fungal infections among others. Inflammatory diseases include autoimmune and rheumatic diseases such as lupus, rheumatoid arthritis, juvenile idiopathic arthritis, and many more. Although Biomeme’s testing pipeline is still in development, there are very many applications where we can make meaningful differences in patients’ lives.
Get the latest tips from Biomeme shipped right to your inbox

Biomeme pioneers a new era in healthcare with the HR-B/V platform. Our host response molecular testing, based on decades of research and collaboration with leading institutions, empowers healthcare...
It’s no secret that there are major issues in the healthcare industry, including breakdowns in doctor-patient communication, delayed test results, and information gaps. Even after consulting with...

Biomeme has announced its participation in a collaborative fight against antimicrobial resistance and antibiotic misuse, focusing on better diagnostic testing. With the Antibacterial Resistance...
401 North Broad St Suite 222 Philadelphia, PA 19108