Written By: Biomeme Staff
EDIT: As RNA viruses like SARS-CoV-2 continue to mutate, so will this panel. Please review our COVID-19 page for the latest information regarding our testing capabilities, including variant/mutation detection.
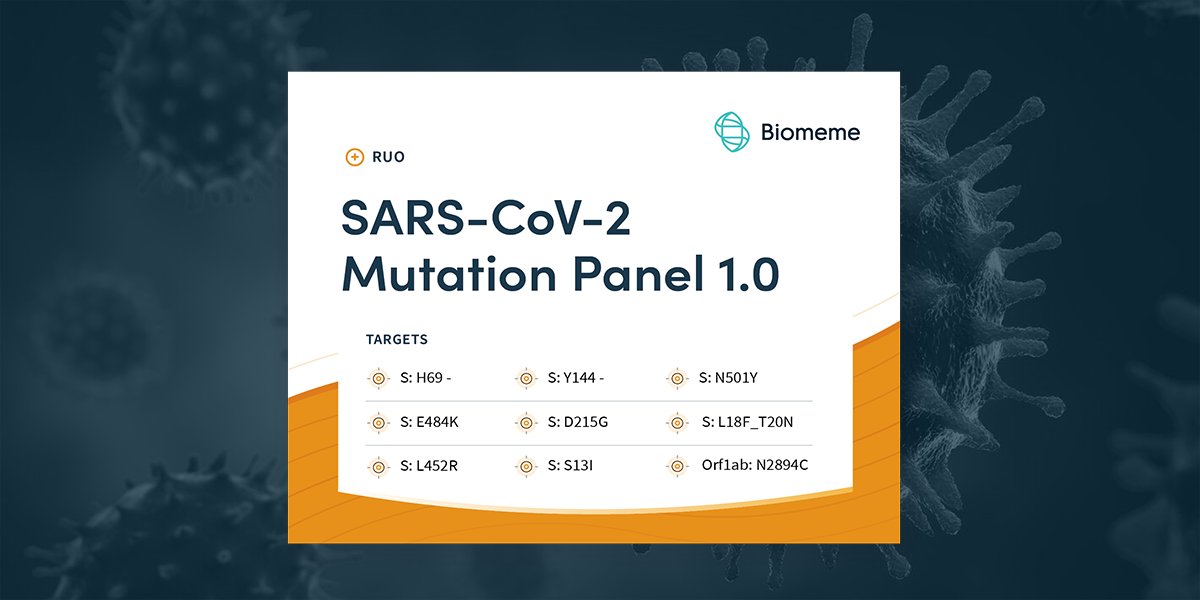
We are proud to announce our new SARS-CoV-2 Mutation Panel 1.0 (RUO) to help researchers, epidemiologists, and local, state, and federal health departments at the forefront of pandemic response detect key mutations and quickly identify the variant associated with those mutations via RT-PCR!
Our SARS-CoV-2 Real-Time RT-PCR Test detects the SARS-CoV-2 Variants of Concern listed below, our new SARS-CoV-2 Mutation Panel 1.0 enables identification of the specific variant of concern by characterizing 9 different loci (the location of a gene in your DNA) within a positive sample, looking for key single point mutations and deletions common to certain variants.
Genetic variants of SARS-CoV-2 have been emerging and circulating around the world throughout the COVID-19 pandemic. All viruses mutate and change, and SARS-CoV-2 is no different. The emergence of variants has created a need for increased genomic surveillance to monitor what the CDC has defined as “Variants of Concern”. These are the variants of SARS-CoV-2 that have shown evidence of an increase in:
Sequences with similar genetic changes are grouped into lineages, and multiple lineages can have the same substitutions. For example, the E484K substitution is found in lineages B.1.351 and P.1. Genomic surveillance efforts provide the capability to more rapidly detect and mitigate the spread of viruses that have increased transmissibility, reduced susceptibility to treatments, etc.
| Mutation | Associated Variant | Biomeme SARS-CoV-2 Test Detection? | |||
| B.1.1.7 20I/501Y.V1 |
B.1.135 20H/501Y.V2 |
P.1 |
B.1.427/9 20C/S:452R |
||
| S: H69 - | |||||
| S: Y144 - | |||||
| S: N501Y | |||||
| S: E484K | |||||
| S: D215G | |||||
| S: L18F_T20N | |||||
| S: L452R | |||||
| S: S13I | |||||
| Orf1ab: N2894C | |||||
If you’re familiar with Biomeme, you already know that all of our assays are pre-mixed and shelf-stable in an effort to simplify and streamline the testing process. If you’re unfamiliar with us or our PCR products, below are the various form factors we manufacture for each of our tests, including our new SARS-CoV-2 Mutation Panel 1.0:
As SARS-CoV-2 continues to evolve, it will be imperative for laboratories and test manufacturers to adapt as well. As new mutations and variants of concern arise, additional mutations will be added to the panel. A single PCR run on the Franklin™ can detect and differentiate up to 27 individual PCR targets. So, starting with 9 mutations, we have lots of room to grow!
Sequencing of novel variants will allow diagnostic tests to stay updated, helping to ensure that new strains are detected. Most importantly, monitoring and sequencing these and other possible variants will ensure that tests, treatments and vaccines maintain efficacy for as long as possible.
Biomeme's SARS-CoV-2 Mutation Panel 1.0 will be generally available beginning Summer 2021 and will be sold on a first come, first serve basis. If you're interested in purchasing, please take a moment to submit this short form and a technical sales rep will follow up with you.
Get the latest tips from Biomeme shipped right to your inbox

Biomeme pioneers a new era in healthcare with the HR-B/V platform. Our host response molecular testing, based on decades of research and collaboration with leading institutions, empowers healthcare...
It’s no secret that there are major issues in the healthcare industry, including breakdowns in doctor-patient communication, delayed test results, and information gaps. Even after consulting with...

Biomeme has announced its participation in a collaborative fight against antimicrobial resistance and antibiotic misuse, focusing on better diagnostic testing. With the Antibacterial Resistance...
401 North Broad St Suite 222 Philadelphia, PA 19108