 Product Updates
Product Updates
Biomeme recently announced the completed acquisition of Predigen. The announcement brings together Predigen's combined decades of host response research with Biomeme's portable PCR test solutions to...
Get the latest tips from Biomeme shipped right to your inbox

New Year, New Biomeme Headquarters We are settled in and so glad to finally welcome our employees, customers, and everyone involved with Biomeme's success to our new headquarters space. To recap, we...

FIND, the global alliance for diagnostics, announced on December 20, 2021 a US$21 million investment to accelerate the development, manufacturing, and launch of affordable, multi-pathogen, molecular...

Through in silico analysis, we’ve confirmed Biomeme’s SARS-Cov-2 assay detects all current variants of concern and variants of interest including delta and omicron. Omicron was first identified in...

Each year, the University City Science Center’s Nucleus Awards bring together the region’s leaders and groundbreakers in innovation, all while supporting Philadelphia’s future as a hub of innovation...

Biomeme is excited to announce that we have signed a definitive agreement to acquire Predigen, a spin-out from Duke University and global leader in the development of host gene expression signatures...

Biomeme’s move into its new corporate headquarters at Netrality's 401 North Broad Street, will begin an exciting new chapter for our company. Our new headquarters were announced earlier this year and...
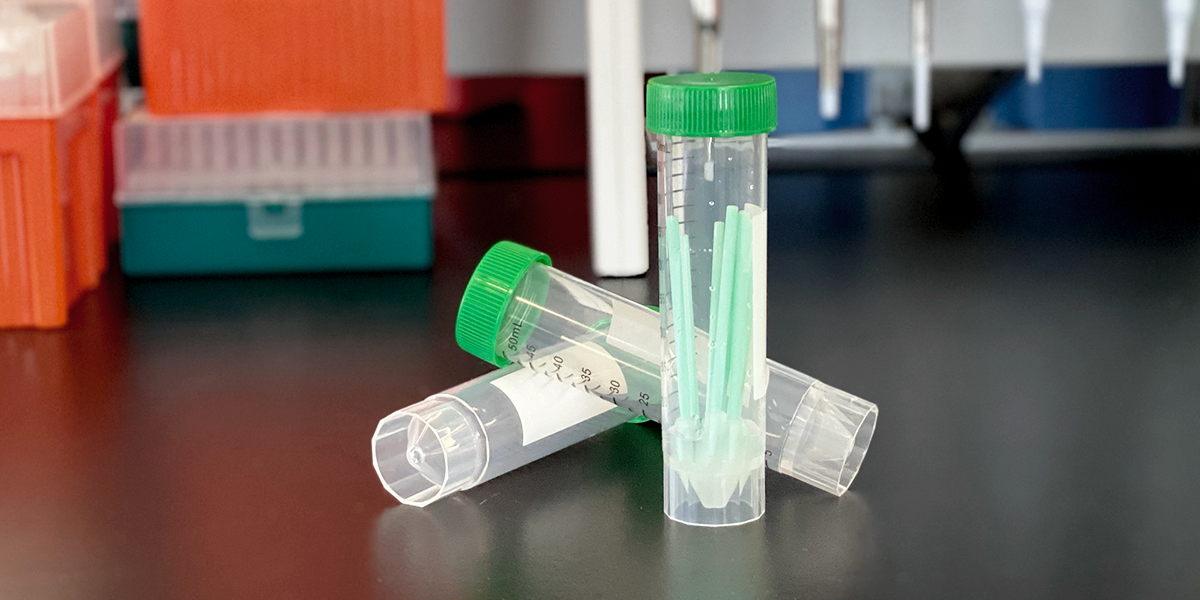
We are pleased to announce that the U.S. Food and Drug Administration (FDA) amended Biomeme’s SARS-CoV-2 Real-Time RT-PCR Test Emergency Use Authorization (EUA) for use with pooled samples in...
.png)
This is a very common question, so let’s get straight to it. There are 2 kinds of tests available for COVID-19: Direct Detection - tells you if portions of the SARS-CoV-2 virus were detected in your...

With support from the Cross-Border Threat Screening and Supply Chain Defense Center of Excellence, Biomeme and Predigen were proud to recently participate in discussion and demonstration of our work...