 Product Updates
Product Updates
We are pleased to announce that the U.S. Food and Drug Administration (FDA) amended Biomeme’s SARS-CoV-2 Real-Time RT-PCR Test Emergency Use Authorization (EUA) for use with pooled samples in...
Get the latest tips from Biomeme shipped right to your inbox
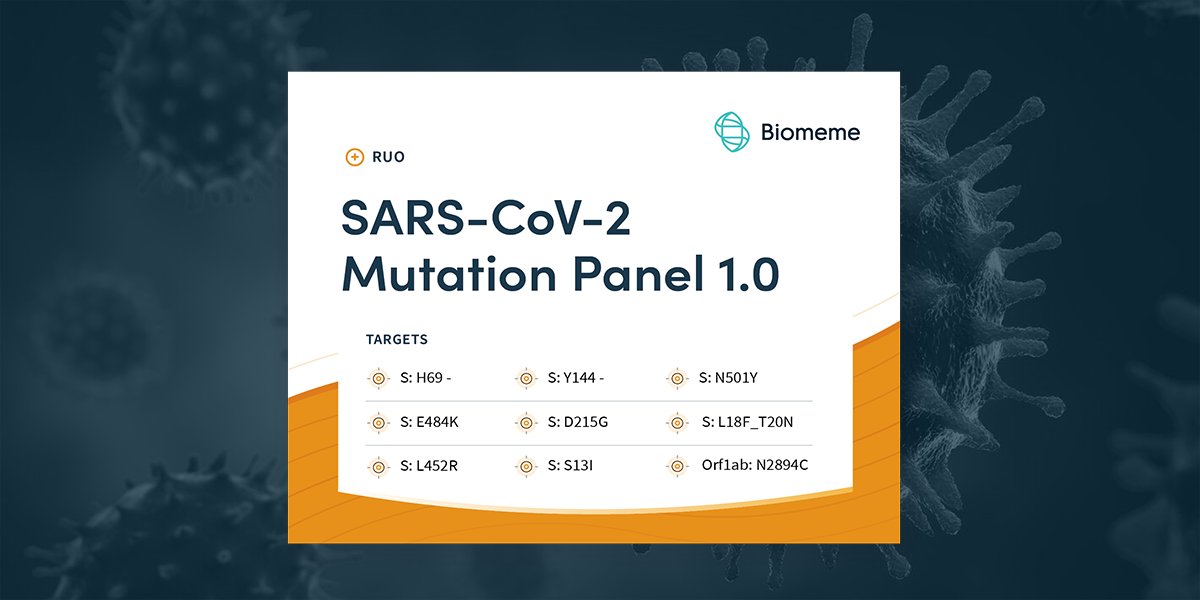
EDIT: As RNA viruses like SARS-CoV-2 continue to mutate, so will this panel. Please review our COVID-19 page for the latest information regarding our testing capabilities, including variant/mutation...

As the world crosses the 1-year mark since the World Health Organization classified COVID-19 as a pandemic, testing is still as crucial as ever. The United States tests an average of 2.78 people per...

The Partnership Back in May 2019, Biomeme and INDICAL Bioscience entered into an exclusive global licensing and research agreement in a joint effort to improve animal health globally. At the time,...
List of SARS-CoV-2 Variants & Classifications Biomeme continuously monitors and analyzes sequences of emerging variants of concern and updates this table accordingly. Variant CDC Label CDC Variant...

When you walk into Biomeme HQ in Philadelphia, you’ll find a bustling office overflowing with our staff, incoming and outgoing packages, and an overworked coffee machine (our office appliances might...
We are pleased to announce that the U.S. Food and Drug Administration (FDA) granted Biomeme’s SARS-CoV-2 Real-Time RT-PCR Test Emergency Use Authorization (EUA) for use in laboratories that meet...

We are proud to announce that Biomeme’s portable test solution to detect SARS-CoV-2 was approved for in vitro diagnostic use by Health Canada on June 30, 2020. Our SARS-CoV-2 Real-Time RT-PCR test...

As the United States rolls into month two of Stay at Home and social distancing orders, ramping up COVID-19 testing is a critical step to track and prevent the spread of the SARS-CoV-2 virus to...
In the time of the virus, it is as important as ever to remain calm and practice preventative measures. At the time of writing, WHO characterized COVID-19 (Coronavirus Disease 2019) as a pandemic and...